Define Definition Of Buffer Capacity
I bought a house as a buffer against inflation. For a given buffer solution there is a working pH range and a defined amount of acid or base that can be neutralized before the pH changes.
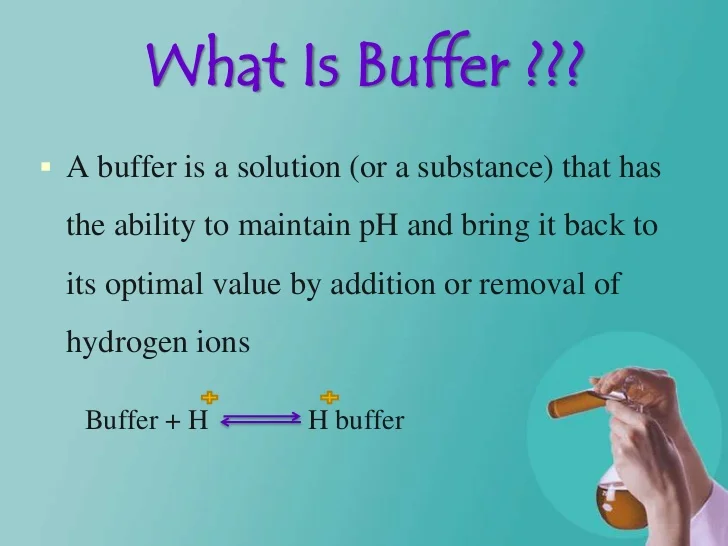
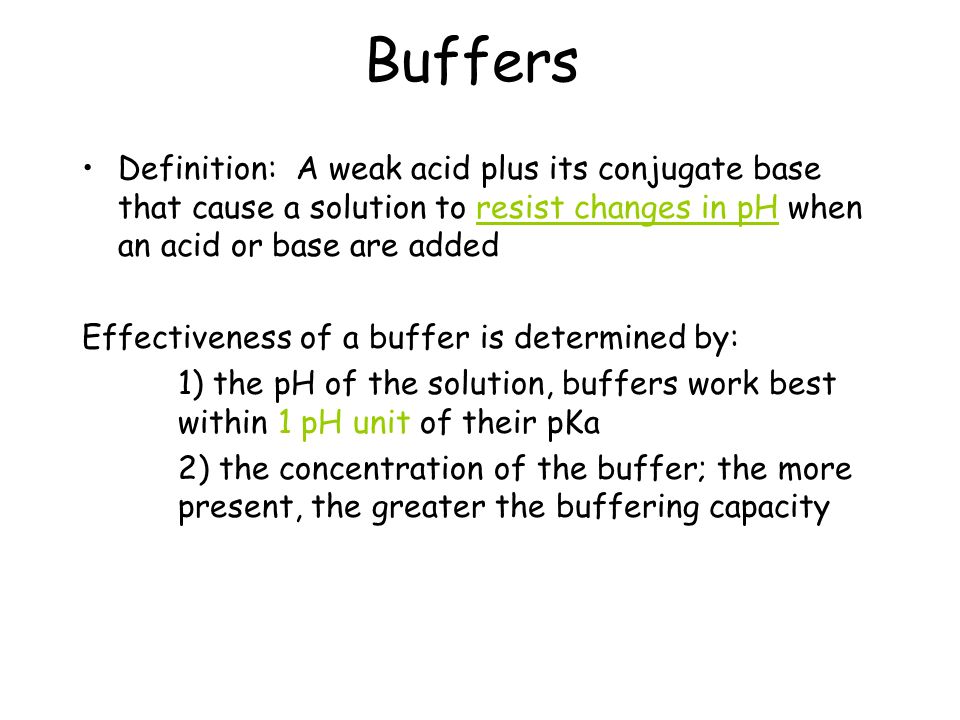
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid.

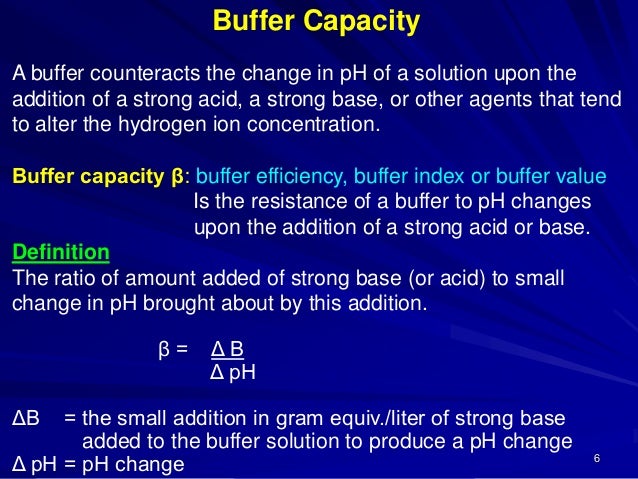
Define definition of buffer capacity. Where n is some equivalents of added strong base per 1 L of the solution. The buffer capacity can also be defined as the amount of mole of strong base needed to change the pH of 1 L of solution by 1 pH of unit. A buffer in circuit design is an amplifier that provides an interface between mismatched circuit elements.
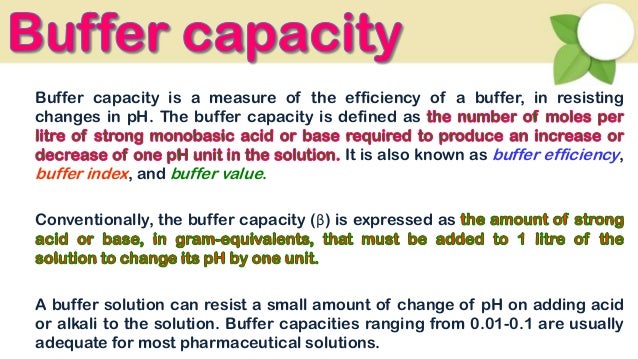
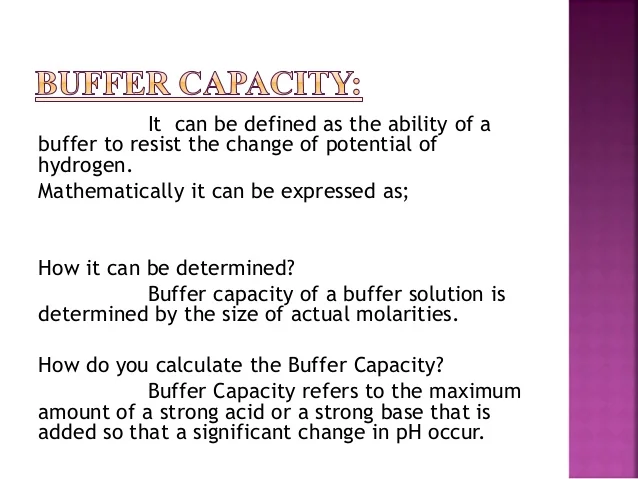
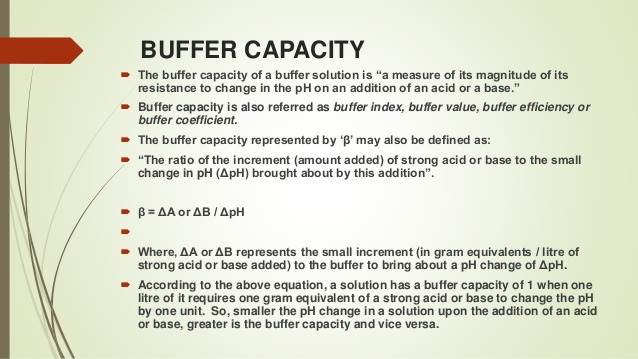
It is the buffers ability to resist change where a buffer is a weak acid or alkaline solution and its conjugate salt. So to give a more clear definition buffer capacity may be defined as the quantity of a strong acid or strong base that must be added to one liter of a solution to change it by one pH unit. Buffer capacity A measure of the resistance of a buffer solution to pH change upon addition or removal of hydroxide ions.
Its PKa value is. A general buffer capacity estimate is 40 percent of the total sum of the molarites of the conjugate base and acid. A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer.
2012 Farlex Inc. The higher the acid concentration of the buffer then the buffer capacity will be higher as well. It is a unitless number.
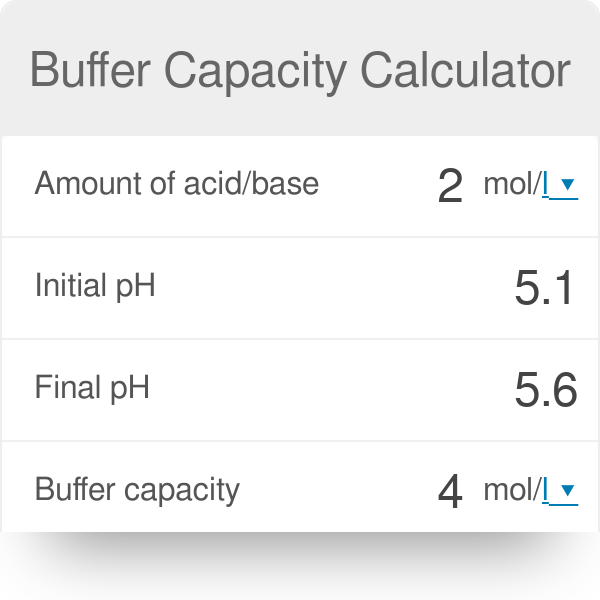
Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. The buffer capacity equation is as follows. McGraw-Hill Dictionary of Scientific Technical Terms 6E Copyright 2003 by The McGraw-Hill Companies Inc.
Something or someone that helps protect from harm. In computer science the term refers to a memory device used for temporary storage. Buffering capacity is defined as the number of moles of strong base or acid needed to change the pH of a liter of buffer solution by one unit.
Also known as buffer efficiency buffer value and buffer index. The amount of acid or base that can be added to a buffer before changing its pH is called its buffer capacity. The Henderson-Hasselbalch equation can be used to measure the approximate pH of a buffer.
Solutions with higher amounts of weak acid have higher levels of buffer capacity when adding a strong base. Solutions with a weaker base have more buffer capacity when adding a strong acid. Buffer definition is - fellow man.
Teo73iStock Getty Images PlusGettyImages. It can be defined as follows. Want to thank TFD for its existence.
The buffer capacity is a quantity in resisting the pH change at the time of addition of an acid or base. Since the concentration of phosphate buffer in the blood plasma is about 8 per cent of that of the bicarbonate buffer its buffering capacity is much lower than bicarbonate in the plasma. Patents-wipo This invention relates to absolute pressure sensors with two bonded wafers containing a pressure sensing structure and forming a reference pressure chamber and a.
How to use buffer in a sentence. Buffering capacity is the effectiveness with which an aqueous solution can absorb and offset the effects of an acid or alkali. Buffer capacity β is defined as the moles of an acid or base necessary to change the pH of a solution by 1 divided by the pH change and the volume of buffer in liters.
The relief manifold assembly preferably includes a buffer vessel defining a buffer chamber in fluid communication with the interior space defined by the fuel tank body. Bəfər kəpasədē chemistry The relative ability of a buffer solution to resist pH change upon addition of an acid or a base. Buffer a chemical substance which has the capacity to bond to H ions removing them from solution when their concentration begins to rise and releasing H ions when their concentration begins to fall.
C the metal parts at the front and back of a train or at the end of a track that help protect the train and reduce damage if the train hits something. A buffer is an aqueous solution used to keep the pH of a solution nearly constant. The phosphate buffer consists of dibasic phosphate HPO 4 and monobasic phosphate H 2 PO 4.
Buffer capacity is a quantitative measure of the resistance to change of pH of a solution by camerino italy containing a buffering agent with respect to a change of acid or alkali concentration. In this way buffers stabilize the pH of biological solutions and are thus important in maintaining HOMEOSTASIS. Buffer capacity is the amount of acid that buffers are capable of absorbing prior to breaking the capacity for adding strong acid.
Note that the addition of n moles of acid will change the pH by the same value but in the opposite direction.
 Equilibrium Constant Relationship Kp Kc Kx Kn Giao Dục
Equilibrium Constant Relationship Kp Kc Kx Kn Giao Dục
Ph Buffers Acids And Bases Introduction To Chemistry
 Exploring Buffers And Buffer Capacity Intro Theory Youtube
Exploring Buffers And Buffer Capacity Intro Theory Youtube
 How To Calculate Buffer Capacity
How To Calculate Buffer Capacity
What Is The Formula For Buffer Capacity Quora
 Pin By Integrated Learning Strategies On Ots Pts And Professionals Social Work Exam Social Work Theories Counseling Psychology
Pin By Integrated Learning Strategies On Ots Pts And Professionals Social Work Exam Social Work Theories Counseling Psychology
 Acid Base Balance And Buffer Systems In The Human Body Ppt Video Online Download
Acid Base Balance And Buffer Systems In The Human Body Ppt Video Online Download
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 What Is Buffer Capacity Youtube
What Is Buffer Capacity Youtube
 We Often Use Automatic Behavioural Scripts To Help Us Address Routines While These Scripts Are Efficient However Emotional Intelligence Psychology Behavior
We Often Use Automatic Behavioural Scripts To Help Us Address Routines While These Scripts Are Efficient However Emotional Intelligence Psychology Behavior
 Buffer Capacity Chemistry Definition And Formula Az Chemistry
Buffer Capacity Chemistry Definition And Formula Az Chemistry
 Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry